Understanding the label
Cosmetic products must include information that explains what they are for and how to use them safely. In line with the Cosmetic Products Regulation, the following information must be included on the label or packaging:
- The name or registered name and address of the Responsible Person
- The content at the time of packaging, by weight or volume
- How long the product will continue to fulfil its function after being opened
- The date of minimum durability (if less than 30 months)
- Any precautions relating to the use of the product
- The manufacturing batch number
- The product’s function, unless it is clear from its presentation
- A list of ingredients, set out in descending order of weight
- The country of origin, for products imported into the EU
What appears on the label?
The name or registered name and address of the Responsible Person
This is the details of the company that places the product on the EU market, usually the manufacturer or importer.
The content at the time of packaging, by weight or volume
The pack and/or label will show the net content of the product, which is its weight or volume excluding the weight of the pack. This is displayed in grams (g) for solid products and millilitres (ml) for liquids. Products that are supplied in very small quantities don’t need to state their weight or volume. These include items containing less than 5 g or 5 ml, single-use packs and free samples.
Many products include the ‘e’ mark. For example ‘200ml e’ may appear on a bottle of shampoo. This shows the product has been filled using the ‘average fill system’, as defined in weights and measures legislation.
The date of minimum durability, or the date the product will continue to fulfil its function until
Products that will last less than 30 months must include a ‘Best before the end of’ date. This is sometimes shown as an egg timer symbol followed by the date.
Products that will last more than 30 months must show a ‘period after opening’ time. This indicates how many months the product will remain in good condition after being used for the first time. It usually takes the form of a symbol of an open cream jar, with the time in months inside or alongside it.
Some products don’t need either of these labels because they won’t deteriorate in normal use. Examples include aerosols, perfumes and single-use packs.
Ingredients
A naming system called the International Nomenclature for Cosmetic Ingredients (INCI) has been developed to make it easy to identify ingredients. The same ingredient names are used in every European country, and most countries worldwide.
Ingredients lists use the same format and convention across products:
- They are headed by the word INGREDIENTS
- Ingredients are listed in order of weight
- Names are taken from the INCI naming system
- For colours, an international naming system called the Colour Index Number (CI Number) is used
Additional enclosed or attached information
A special symbol is used to indicate if additional information about the product is either enclosed separately or attached. The symbol is generally used when there is not enough space to show all the information on the packaging.
Recycling
All companies in Europe have a legal obligation to recycle and recover packaging waste, usually via a specialist company. The most common recycling symbol on cosmetic products is the ‘Green Dot’. This is a trademark that shows membership of a specific recycling and recovery scheme to deal with the packaging waste of a company’s products.

Different product types
Sunscreen labelling
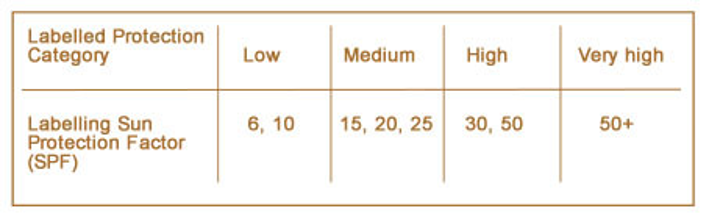
Labelling on sunscreen products includes a Sun Protection Factor (SPF) rating. This shows how much protection the product provides against UVB light. The SPF rating system was introduced by the cosmetics industry and is used throughout Europe and in many other parts of the world. It shows the ability of a sun protection product to filter out UVB rays. For example, an SPF of 15 will filter out approximately 93% of UVB rays and an SPF of 30 will filter out around 96%.
Choosing a sunscreen that provides both UVA and UVB protection is highly recommended. The letters “UVA” in a circle are used to show that a product contains at least the recommended minimum level of UVA protection for a sunscreen product.
The most common SPF numbers and the categories they fall into are shown in the table below.
Hair dye labelling
Labelling for hair dye products includes additional information, as some people are allergic to these products. Warnings and safety instructions are included on both the outer pack and instruction leaflets for hair colourants. These explain that all users should carry out an allergy alert test, even if they have used hair colour before. The instructions also explain how to carry out the test.
Find out more about hair dyeing at Colour Well, Colour Wise.

Labelling of children’s toothpaste
Toothpastes for children are another category of products that include special labelling. Fluoride in toothpaste improves dental health and has been shown to be safe for children. EU regulations stipulate the maximum amount of fluoride that can be included. Member States have also issued guidelines for recommended fluoride levels in toothpastes for children. Reflecting this, children’s toothpaste is labelled with a recommended quantity and instructions for use.